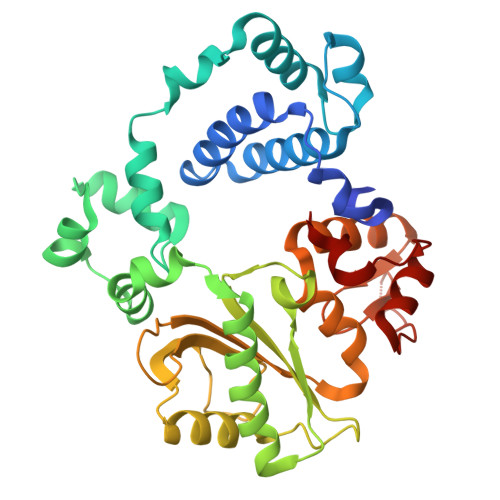
Nonhomologous end-joining uses distinct mechanisms to repair each strand of a double strand break.
Luthman, A.J., Chiruvella, K.K., Kaminski, A.M., Kunkel, T.A., Pedersen, L.C., Ramsden, D.A.(2025) Nat Commun 16: 11599-11599
- PubMed: 41285786
- DOI: https://doi.org/10.1038/s41467-025-66528-8
- Primary Citation of Related Structures:
9NPU - PubMed Abstract:
Nonhomologous end-joining repairs chromosomal double strand breaks, but it is unknown whether both strands are repaired by this pathway, and if one strand break's repair path impacts the other. Here, we show that nonhomologous end-joining employs both of two a priori possible strategies. Strand breaks that can be directly ligated are joined near-simultaneously, with no effect of one strand break's repair path on the other. More complex end structures require obligatorily ordered repair. The first strand to be repaired is used as template for repair of the opposite/second strand break, with the latter repair reaction occurring fastest when also coupled to nonhomologous end-joining. Enforced asymmetry in repair of each strand break can extend to the gap-filling polymerase employed, and whether the polymerases incorporate RNA or DNA. Our results resolve questions about pathway mechanism and identify a requirement for flexibility of the nonhomologous end-joining machinery for efficient repair of both strand breaks within diverse cellular double strand breaks.
- Department of Biochemistry and Biophysics, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Organizational Affiliation: