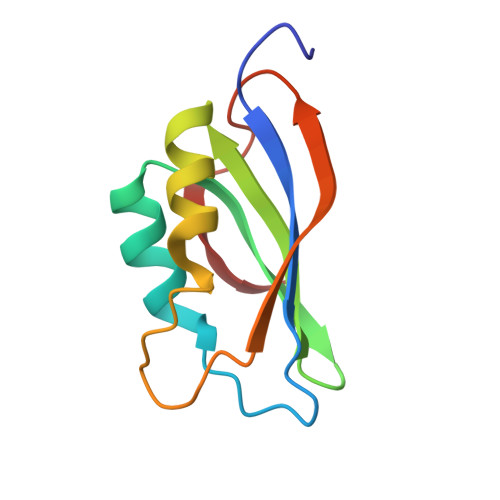
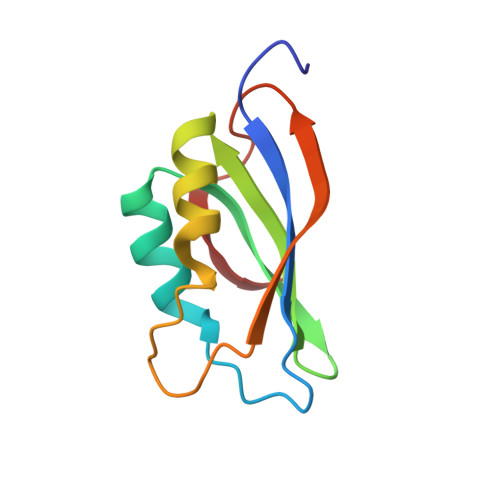
Three-dimensional structure of acylphosphatase. Refinement and structure analysis.
Pastore, A., Saudek, V., Ramponi, G., Williams, R.J.(1992) J Mol Biology 224: 427-440
- PubMed: 1313885
- DOI: https://doi.org/10.1016/0022-2836(92)91005-a
- Primary Citation of Related Structures:
1APS - PubMed Abstract:
We report here the complete determination of the solution structure of acylphosphatase, a small enzyme that catalyses the hydrolysis of organic acylphosphates, as determined by distance geometry methods based on nuclear magnetic resonance information. A non-standard strategy for the distance geometry calculations was used and is described here some detail. The five best structures were then refined by restrained energy minimization and molecular dynamics in order to explore the conformational space consistent with the experimental data. We address the question of whether the solution structure of acylphosphatase follows the general principles of protein structure, i.e. those learned from analysing crystal structures. Static and dynamic features are discussed in detail. An uncommon beta-alpha-beta motif, so far found only in procarboxypeptidase B and in an RNA-binding protein, is present in acylphosphatase.
Organizational Affiliation:
EMBL, Heidelberg, Germany.