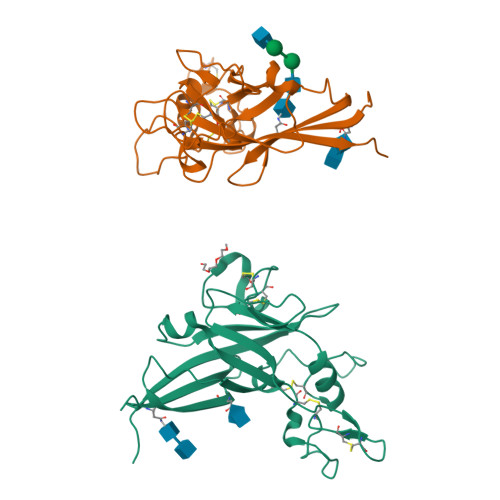
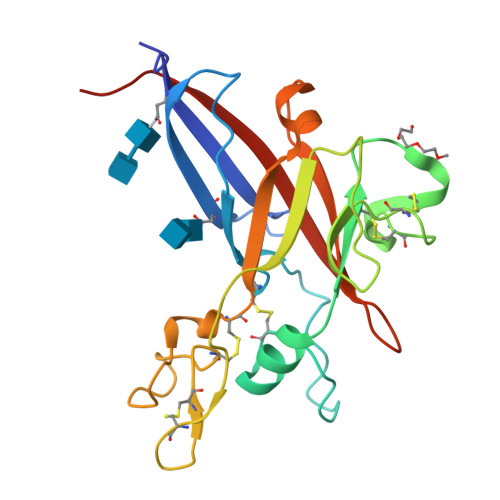
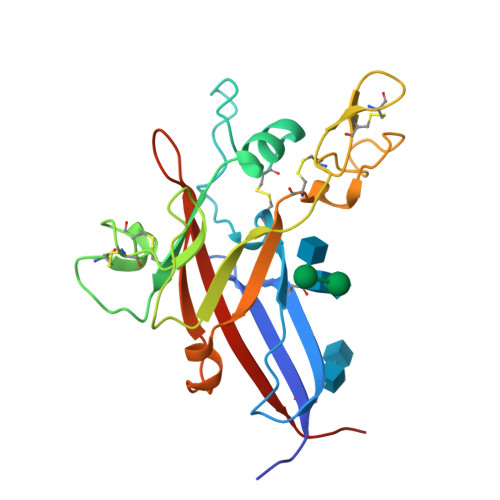
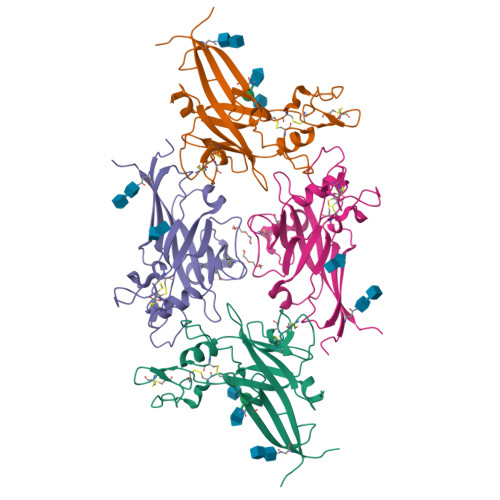
Structure and Mechanism of a Coreceptor for Infection by a pathogenic feline retrovirus
Barnett, A.L., Wensel, D.L., Li, W., Fass, D., Cunningham, J.M.(2003) J Virol 77: 2717-2729
- PubMed: 12552012
- DOI: https://doi.org/10.1128/jvi.77.4.2717-2729.2003
- Primary Citation of Related Structures:
1LCS - PubMed Abstract:
Infection of T lymphocytes by the cytopathic retrovirus feline leukemia virus subgroup T (FeLV-T) requires FeLIX, a cellular coreceptor that is encoded by an endogenous provirus and closely resembles the receptor-binding domain (RBD) of feline leukemia virus subgroup B (FeLV-B). We determined the structure of FeLV-B RBD, which has FeLIX activity, to a 2.5-A resolution by X-ray crystallography. The structure of the receptor-specific subdomain of this glycoprotein differs dramatically from that of Friend murine leukemia virus (Fr-MLV), which binds a different cell surface receptor. Remarkably, we find that Fr-MLV RBD also activates FeLV-T infection of cells expressing the Fr-MLV receptor and that FeLV-B RBD is a competitive inhibitor of infection under these conditions. These studies suggest that FeLV-T infection relies on the following property of mammalian leukemia virus RBDs: the ability to couple interaction with one of a variety of receptors to the activation of a conserved membrane fusion mechanism. A comparison of the FeLV-B and Fr-MLV RBD structures illustrates how receptor-specific regions are linked to conserved elements critical for postbinding events in virus entry.
Organizational Affiliation:
Department of Medicine, Howard Hughes Medical Institute, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA.