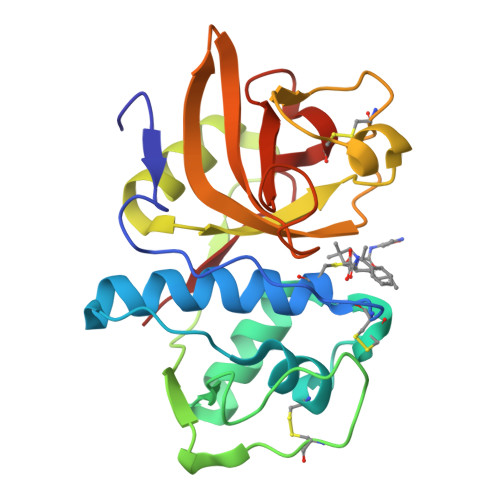
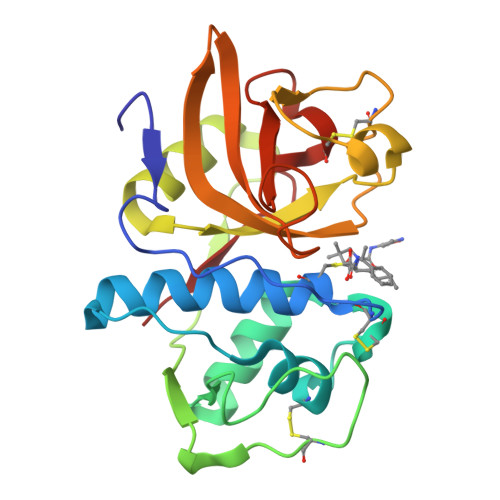
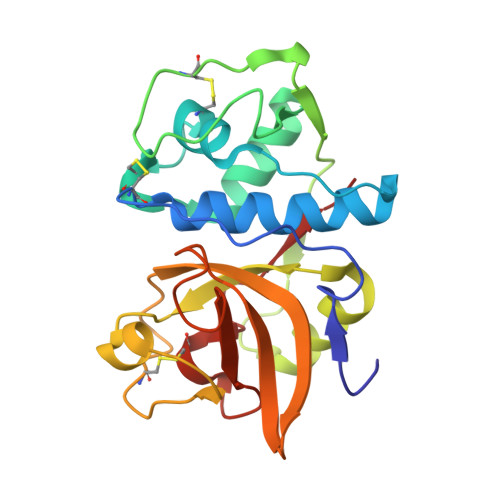
Novel, potent P2-P3 pyrrolidine derivatives of ketoamide-based cathepsin K inhibitors.
Barrett, D.G., Catalano, J.G., Deaton, D.N., Hassell, A.M., Long, S.T., Miller, A.B., Miller, L.R., Ray, J.A., Samano, V., Shewchuk, L.M., Wells-Knecht, K.J., Willard Jr., D.H., Wright, L.L.(2006) Bioorg Med Chem Lett 16: 1735-1739
- PubMed: 16376075
- DOI: https://doi.org/10.1016/j.bmcl.2005.11.101
- Primary Citation of Related Structures:
2BDL - PubMed Abstract:
Starting from a potent pantolactone ketoamide cathepsin K inhibitor discovered from structural screening, conversion of the lactone scaffold to a pyrrolidine scaffold allowed exploration of the S(3) subsite of cathepsin K. Manipulation of P3 and P1' groups afforded potent inhibitors with drug-like properties.
Organizational Affiliation:
Department of Medicinal Chemistry, GlaxoSmithKline, Research Triangle Park, NC 27709, USA.