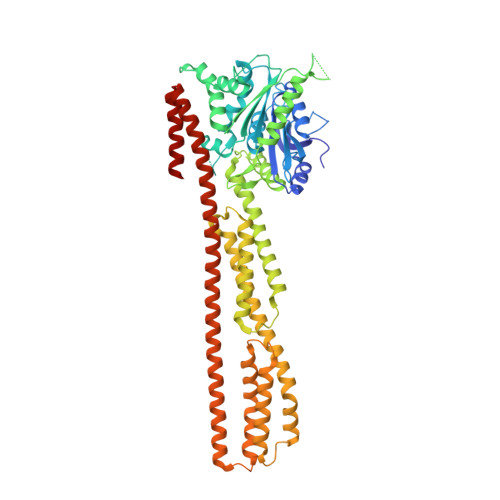
Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins.
Prakash, B., Praefcke, G.J., Renault, L., Wittinghofer, A., Herrmann, C.(2000) Nature 403: 567-571
- PubMed: 10676968
- DOI: https://doi.org/10.1038/35000617
- Primary Citation of Related Structures:
1DG3 - PubMed Abstract:
Interferon-gamma is an immunomodulatory substance that induces the expression of many genes to orchestrate a cellular response and establish the antiviral state of the cell. Among the most abundant antiviral proteins induced by interferon-gamma are guanylate-binding proteins such as GBP1 and GBP2. These are large GTP-binding proteins of relative molecular mass 67,000 with a high-turnover GTPase activity and an antiviral effect. Here we have determined the crystal structure of full-length human GBP1 to 1.8 A resolution. The amino-terminal 278 residues constitute a modified G domain with a number of insertions compared to the canonical Ras structure, and the carboxy-terminal part is an extended helical domain with unique features. From the structure and biochemical experiments reported here, GBP1 appears to belong to the group of large GTP-binding proteins that includes Mx and dynamin, the common property of which is the ability to undergo oligomerization with a high concentration-dependent GTPase activity.
- Max-Planck-Institut für Molekulare Physiologie, Dortmund, Germany.
Organizational Affiliation: