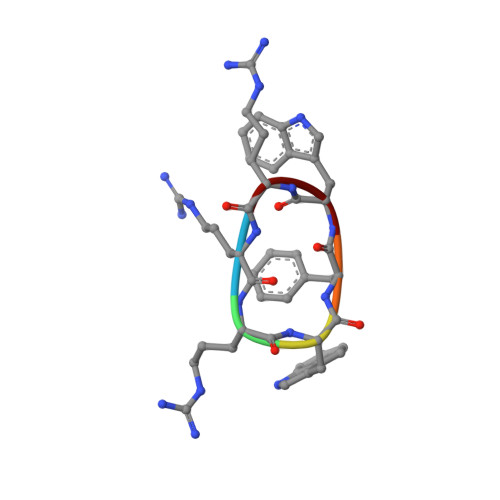
Structures of cyclic, antimicrobial peptides in a membrane-mimicking environment define requirements for activity.
Appelt, C., Wessolowski, A., Dathe, M., Schmieder, P.(2007) J Pept Sci 14: 524-527
- PubMed: 17985394
- DOI: https://doi.org/10.1002/psc.924
- Primary Citation of Related Structures:
2OTQ, 2OX2 - PubMed Abstract:
New antimicrobial compounds are of major importance because of the growing problem of bacterial resistance. In this context, antimicrobial peptides have received a lot of attention. Their mechanism of action, however, is often obscure. Here, the structures of two cyclic, antimicrobial peptides from the family of arginine- and tryptophan-rich peptides determined in a membrane-mimicking environment are described. The sequence of the peptides has been obtained from a cyclic parent peptide by scrambling the amino acids. While the activity of the peptides is similar to that of the parent peptide, the structures are not. The peptides do, however, all adopt an amphiphilic structure. A comparison between the structures helps to define the requirements for the activity of these peptides.
- Leibnizinstitut für Molekulare Pharmakologie (FMP), Robert-Rössle-Str. 10, D-13125 Berlin, Germany.
Organizational Affiliation: