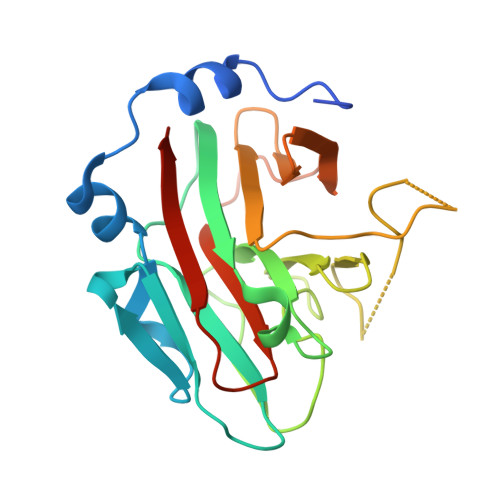
SPRY domain-containing SOCS box protein 2: crystal structure and residues critical for protein binding.
Kuang, Z., Yao, S., Xu, Y., Lewis, R.S., Low, A., Masters, S.L., Willson, T.A., Kolesnik, T.B., Nicholson, S.E., Garrett, T.J., Norton, R.S.(2009) J Mol Biology 386: 662-674
- PubMed: 19154741
- DOI: https://doi.org/10.1016/j.jmb.2008.12.078
- Primary Citation of Related Structures:
3EK9 - PubMed Abstract:
The four mammalian SPRY (a sequence repeat in dual-specificity kinase splA and ryanodine receptors) domain-containing suppressor of cytokine signalling (SOCS) box proteins (SSB-1 to -4) are characterised by a C-terminal SOCS box and a central SPRY domain. The latter is a protein interaction module found in over 1600 proteins, with more than 70 encoded in the human genome. Here we report the crystal structure of the SPRY domain of murine SSB-2 and compare it with the SSB-2 solution structure and crystal structures of other B30.2/SPRY domain-containing family proteins. The structure is a bent beta-sandwich, consisting of two seven-stranded beta-sheets wrapped around a long loop that extends from the centre strands of the inner or concave beta-sheet; it closely matches those of GUSTAVUS and SSB-4. The structure is also similar to those of two recently determined Neuralized homology repeat (NHR) domains (also known as NEUZ domains), with detailed comparisons, suggesting that the NEUZ/NHR domains form a subclass of SPRY domains. The binding site on SSB-2 for the prostate apoptosis response-4 (Par-4) protein has been mapped in finer detail using mutational analyses. Moreover, SSB-1 was shown to have a Par-4 binding surface similar to that identified for SSB-2. Structural perturbations of SSB-2 induced by mutations affecting its interaction with Par-4 and/or c-Met have been characterised by NMR. These comparisons, in conjunction with previously published dynamics data from NMR relaxation studies and coarse-grained dynamics simulation using normal mode analysis, further refine our understanding of the structural basis for protein recognition of SPRY domain-containing proteins.
- The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria 3052, Australia.
Organizational Affiliation: