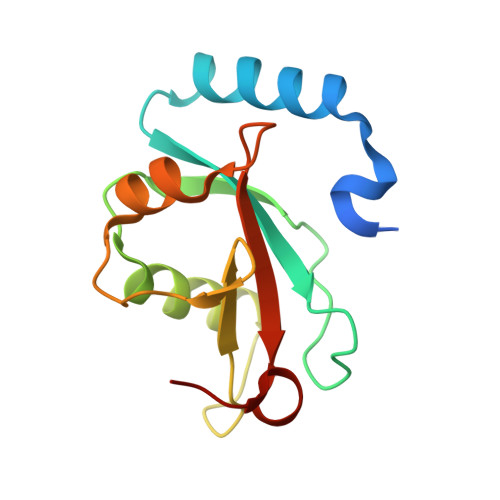
FYCO1 Contains a C-terminally Extended, LC3A/B-preferring LC3-interacting Region (LIR) Motif Required for Efficient Maturation of Autophagosomes during Basal Autophagy
Olsvik, H.L., Lamark, T., Takagi, K., Larsen, K.B., Evjen, G., vervatn, A., Mizushima, T., Johansen, T.(2015) J Biological Chem 290: 29361-29374
- PubMed: 26468287
- DOI: https://doi.org/10.1074/jbc.M115.686915
- Primary Citation of Related Structures:
5D94 - PubMed Abstract:
FYCO1 (FYVE and coiled-coil protein 1) is a transport adaptor that binds to phosphatidylinositol 3-phosphate, to Rab7, and to LC3 (microtubule-associated protein 1 light chain 3) to mediate transport of late endosomes and autophagosomes along microtubules in the plus end direction. We have previously shown that FYCO1 binds to LC3B via a 19-amino acid sequence containing a putative core LC3-interacting region (LIR) motif. Here, we show that FYCO1 preferentially binds to LC3A and -B. By peptide array-based two-dimensional mutational scans of the binding to LC3B, we found FYCO1 to contain a C-terminally extended LIR domain. We determined the crystal structure of a complex between a 13-amino acid LIR peptide from FYCO1 and LC3B at 1.53 Å resolution. By combining the structural information with mutational analyses, both the basis for the C-terminally extended LIR and the specificity for LC3A/B binding were revealed. FYCO1 contains a 9-amino acid-long F-type LIR motif. In addition to the canonical aromatic residue at position 1 and the hydrophobic residue at position 3, an acidic residue and a hydrophobic residue at positions 8 and 9, respectively, are important for efficient binding to LC3B explaining the C-terminal extension. The specificity for binding to LC3A/B is due to the interaction between Asp(1285) in FYCO1 and His(57) in LC3B. To address the functional significance of the LIR motif of FYCO1, we generated FYCO1 knock-out cells that subsequently were reconstituted with GFP-FYCO1 WT and LIR mutant constructs. Our data show that FYCO1 requires a functional LIR motif to facilitate efficient maturation of autophagosomes under basal conditions, whereas starvation-induced autophagy was unaffected.
- From the Molecular Cancer Research Group, Institute of Medical Biology, University of Tromsø - The Arctic University of Norway, 9037 Tromsø, Norway and.
Organizational Affiliation: