Insights into Heptosyltransferase I Catalysis and Inhibition through the Structure of Its Ternary Complex.
Blaukopf, M., Worrall, L., Kosma, P., Strynadka, N.C.J., Withers, S.G.(2018) Structure 26: 1399-1407.e5
- PubMed: 30122450
- DOI: https://doi.org/10.1016/j.str.2018.07.001
- Primary Citation of Related Structures:
6DFE - PubMed Abstract:
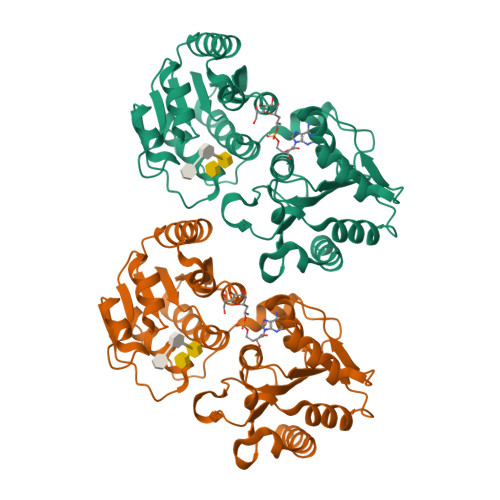
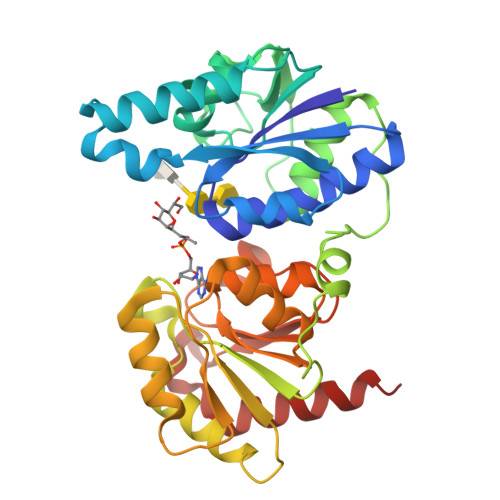
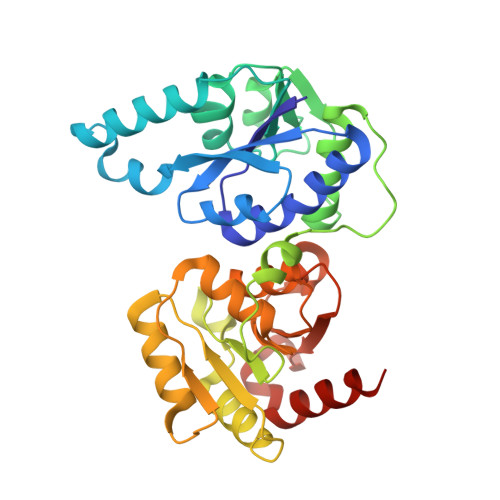
Heptosyltransferase I (WaaC) is a highly conserved glycosyltransferase found in Gram-negative bacteria that transfers a heptose residue onto the endotoxin inner core structure (ReLPS) of the outer membrane. Knockouts of WaaC have decreased virulence and increased susceptibility to antibiotics, making WaaC a potential drug target. While previous studies have elucidated the structure of the holoenzyme and a donor analog complex, no information on the binding mode of the acceptor has been available so far. By soaking of a chemically modified functional acceptor, along with a stable donor analog, the crystal structure of a pseudo-ternary complex of WaaC was obtained at 2.3-Å resolution. The acceptor is bound in an unusual horseshoe conformation stabilized by interaction of the anionic carboxylate and phosphate groups at its center and tips with highly conserved Lys and Arg residues. This binding is accompanied by both inter- and intra-domain movements within the protein.
Organizational Affiliation:
University of Natural Resources and Life Sciences - Vienna, Department of Chemistry, Muthgasse 18, 1190 Vienna, Austria. Electronic address: markus.blaukopf@boku.ac.at.