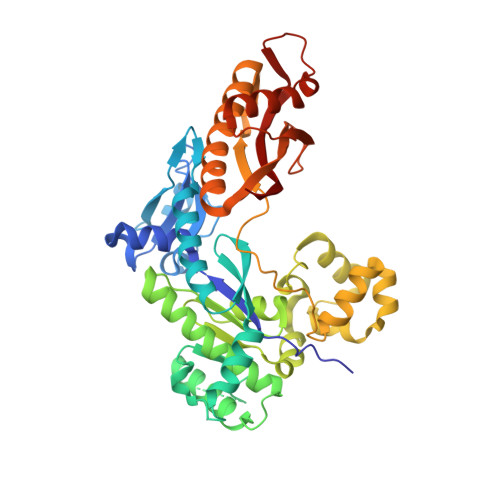
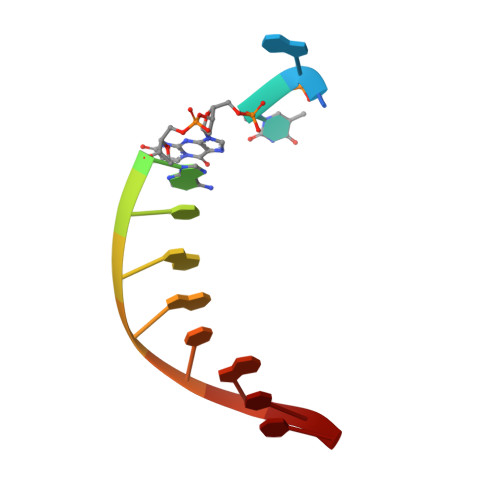
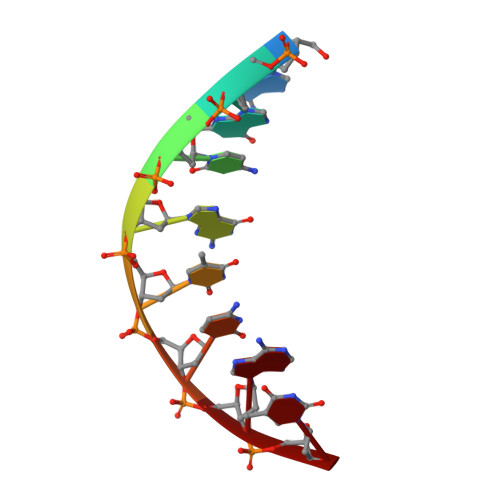
The peroxidation-derived DNA adduct, 6-oxo-M 1 dG, is a strong block to replication by human DNA polymerase eta.
Richie-Jannetta, R., Pallan, P., Kingsley, P.J., Kamdar, N., Egli, M., Marnett, L.J.(2023) J Biological Chem 299: 105067-105067
- PubMed: 37468099
- DOI: https://doi.org/10.1016/j.jbc.2023.105067
- Primary Citation of Related Structures:
8EVE, 8EVF - PubMed Abstract:
The DNA adduct 6-oxo-M 1 dG, (3-(2'-deoxy-β-D-erythro-pentofuranosyl)-6-oxo-pyrimido(1,2alpha)purin-10(3H)-one) is formed in the genome via oxidation of the peroxidation-derived adduct M 1 dG. However, the effect of 6-oxo-M 1 dG adducts on subsequent DNA replication is unclear. Here we investigated the ability of the human Y-family polymerase hPol η to bypass 6-oxo-M 1 dG. Using steady-state kinetics and analysis of DNA extension products by liquid chromatography-tandem mass spectrometry, we found hPol η preferentially inserts a dAMP or dGMP nucleotide into primer-templates across from the 6-oxo-M 1 dG adduct, with dGMP being slightly preferred. We also show primer-templates with a 3'-terminal dGMP or dAMP across from 6-oxo-M 1 dG were extended to a greater degree than primers with a dCMP or dTMP across from the adduct. In addition, we explored the structural basis for bypass of 6-oxo-M 1 dG by hPol η using X-ray crystallography of both an insertion-stage and an extension-stage complex. In the insertion-stage complex, we observed that the incoming dCTP opposite 6-oxo-M 1 dG, although present during crystallization, was not present in the active site. We found the adduct does not interact with residues in the hPol η active site but rather forms stacking interactions with the base pair immediately 3' to the adduct. In the extension-stage complex, we observed the 3' hydroxyl group of the primer strand dGMP across from 6-oxo-M 1 dG is not positioned correctly to form a phosphodiester bond with the incoming dCTP. Taken together, these results indicate 6-oxo-M 1 dG forms a strong block to DNA replication by hPol η and provide a structural basis for its blocking ability.
- A. B. Hancock, Jr, Memorial Laboratory for Cancer Research, Departments of Biochemistry, Chemistry and Pharmacology, Vanderbilt-Ingram Cancer Center, Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
Organizational Affiliation: