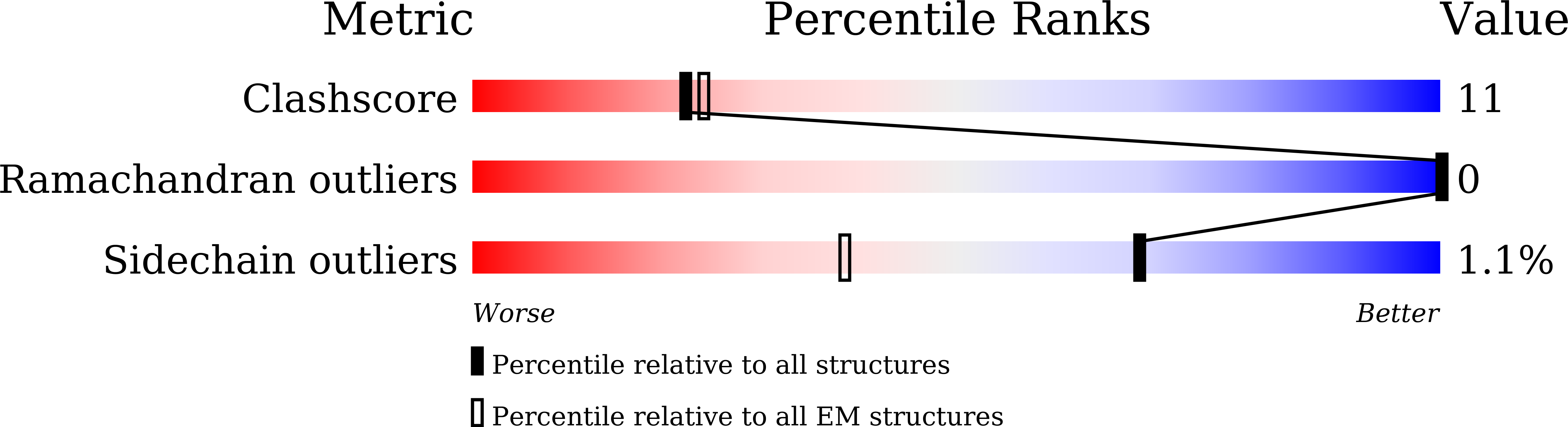Structural insights into the mechanisms of urea permeation and distinct inhibition modes of urea transporters.
Huang, S.M., Huang, Z.Z., Liu, L., Xiong, M.Y., Zhang, C., Cai, B.Y., Wang, M.W., Cai, K., Jia, Y.L., Wang, J.L., Zhang, M.H., Xie, Y.H., Li, M., Zhang, H., Weng, C.H., Wen, X., Li, Z., Sun, Y., Yi, F., Yang, Z., Xiao, P., Yang, F., Yu, X., Tie, L., Yang, B.X., Sun, J.P.(2024) Nat Commun 15: 10226-10226
- PubMed: 39587082
- DOI: https://doi.org/10.1038/s41467-024-54305-y
- Primary Citation of Related Structures:
8XD7, 8XD9, 8XDA, 8XDB, 8XDC, 8XDD, 8XDE, 8XDF, 8XDG, 8XDH, 8XDI - PubMed Abstract:
Urea's transmembrane transport through urea transporters (UT) is a fundamental physiological behavior for life activities. Here, we present 11 cryo-EM structures of four UT members in resting states, urea transport states, or inactive states bound with synthetic competitive, uncompetitive or noncompetitive inhibitor. Our results indicate that the binding of urea via a conserved urea recognition motif (URM) and the urea transport via H-bond transfer along the Q Pb -T 5b -T 5a -Q Pa motif among different UT members. Moreover, distinct binding modes of the competitive inhibitors 25a and ATB3, the uncompetitive inhibitor CF11 and the noncompetitive inhibitor HQA2 provide different mechanisms for blocking urea transport and achieved selectivity through L-P pocket, UCBP region and SCG pocket, respectively. In summary, our study not only allows structural understanding of urea transport via UTs but also afforded a structural landscape of hUT-A2 inhibition by competitive, uncompetitive and noncompetitive inhibitors, which may facilitate developing selective human UT-A inhibitors as a new class of salt-sparing diuretics.
Organizational Affiliation:
Department of Physiology and Pathophysiology, School of Basic Medical Sciences, State Key Laboratory of Vascular Homeostasis and Remodeling, Beijing Key Laboratory of Cardiovascular Receptors Research, Peking University, Beijing, China.