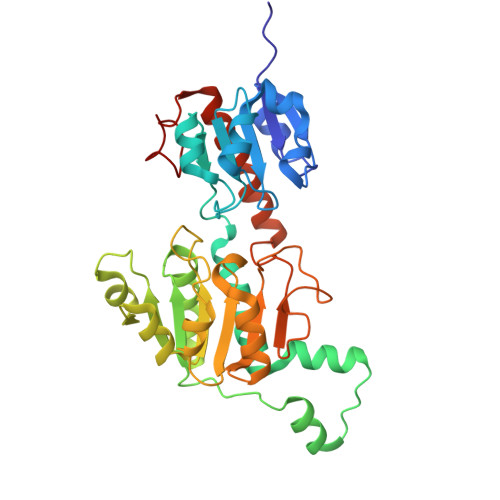
CtBP1/2 oligomerization promotes G9a-Mediated transcriptional repression.
Zhang, B., Jiang, J., Sun, W., Hu, S., Chen, P., Li, L., Jiang, M., Chen, J., Zeng, J., Cai, D., Luo, Q., Liu, W., Cai, Q., Chen, S.(2025) J Biological Chem 302: 111063-111063
- PubMed: 41419197
- DOI: https://doi.org/10.1016/j.jbc.2025.111063
- Primary Citation of Related Structures:
9WRI - PubMed Abstract:
Corepressors CtBP1 and CtBP2 (CtBP1/2) are evolutionarily conserved transcriptional regulators that repress gene expression by recruiting chromatin modifiers, yet the structural basis of this process remains elusive. Here, we identify a direct interaction between CtBP1/2 and the histone H3 lysine 9 (H3K9) methyltransferase G9a. Crystallographic and biochemical analyses reveal that a CtBP1/2 tetramer simultaneously engages two G9a molecules through a motif within the pre-SET domain of G9a, which is absent in its paralog GLP. This interaction enhances G9a catalytic activity in a manner strictly dependent on the oligomeric state of CtBP1/2. Disruption of CtBP2 tetramerization diminishes its association with G9a and abolishes enzymatic activation, underscoring the functional importance of CtBP1/2 oligomerization. In colorectal cancer (CRC) cells, CtBP2 and G9a co-occupy the PTEN promoter, where disruption of their interface reduces H3K9me2 deposition, derepresses PTEN expression, attenuates PI3K-AKT signaling, and impairs CRC cell proliferation. Together, these findings establish a structural framework for CtBP-mediated regulation of G9a activity and highlight the CtBP1/2-G9a complex as a potential therapeutic target in colorectal cancer.
- School of Pharmaceutical Sciences, Fujian Provincial Key Laboratory of Innovative Drug Target Research, Xiamen University, Xiamen, China.
Organizational Affiliation: