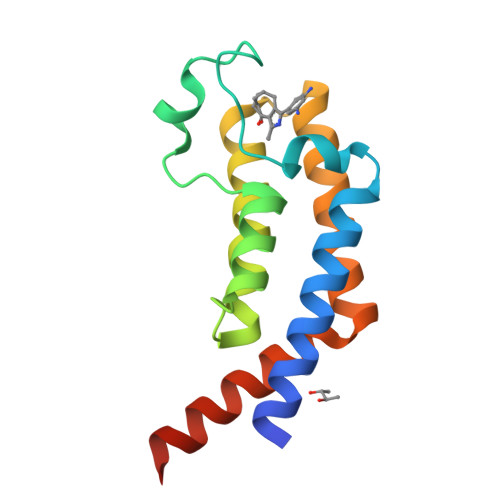
A novel inhibitor against the Bromodomain Protein 1 of the malaria pathogen Plasmodium falciparum.
Amann, M., Warstat, R., Rechten, K.K., Theuer, P., Schustereder, M., Clavey, S., Breit, B., Einsle, O., Hugle, M., Petter, M., Gunther, S.(2025) ChemMedChem : e202500024-e202500024
- PubMed: 40099623
- DOI: https://doi.org/10.1002/cmdc.202500024
- Primary Citation of Related Structures:
9HGF, 9HH7, 9HH8, 9HHA, 9HHB, 9HHC, 9HHD - PubMed Abstract:
The rise of drug resistances in malaria necessitates the exploration of novel therapeutic strategies. Targeting epigenetic pathways could open new, promising treatment avenues. In this study, we focus on the essential Bromodomain Protein 1 (PfBDP1) of the malaria pathogen Plasmodium falciparum. Utilizing the pan-selective bromodomain inhibitor MPM6, we identified a potent initial hit and subsequently developed it into a nanomolar binder. Through a combination of virtual docking, isothermal titration calorimetry, and X-ray crystallography, we elucidated the molecular interactions of the new inhibitors with the bromodomain (BRD) of the protein (PfBDP1-BRD). Our findings include the first co-crystallized inhibitors with the structures of PfBRD1-BRD as well as the bromodomain of the close homologous protein of Plasmodium vivax (PvBDP1-BRD). The structures provide new insights into their binding mechanisms. Further validation using conditional knockdown of PfBDP1 in P. falciparum demonstrated parasite sensitivity to the inhibitor, underscoring its potential in a targeted therapeutic approach against malaria.
Organizational Affiliation:
Albert-Ludwigs-Universität Freiburg: Albert-Ludwigs-Universitat Freiburg, Institut für pharmazeutische Wissenschaften, GERMANY.