Integrating Surface Plasmon Resonance and Docking Analysis for Mechanistic Insights of Tryptase Inhibitors.
Porta, A., Manelfi, C., Talarico, C., Beccari, A.R., Brindisi, M., Summa, V., Iaconis, D., Gobbi, M., Beeg, M.(2025) Molecules 30
- PubMed: 40142113
- DOI: https://doi.org/10.3390/molecules30061338
- Primary Citation of Related Structures:
9QFU, 9QFV - PubMed Abstract:
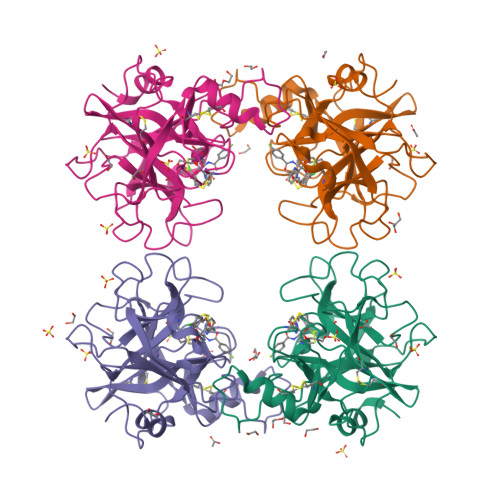
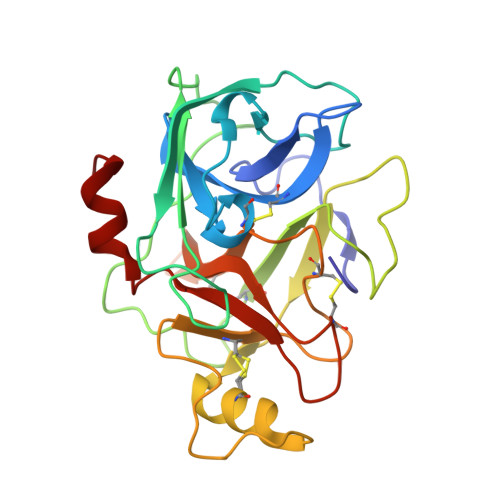
Tryptase is a tetrameric serine protease and a key component of mast cell granules. Here, we explored an integrated approach to characterize tryptase ligands, combining novel experimental binding studies using Surface Plasmon Resonance, with in silico analysis through the Exscalate platform. For this, we focused on three inhibitors previously reported in the literature, including a bivalent inhibitor and its corresponding monovalent compound. All three ligands showed concentration-dependent binding to immobilized human tryptase with the bivalent inhibitor showing the highest affinity. Furthermore, Rmax values were similar, indicating that the compounds occupy all four binding pockets of the tryptase tetramer. This hypothesis was supported by in silico computational analysis that revealed the binding mode of the monovalent ligand, one in each monomer pocket, compared with crystal structure of the bivalent one, which simultaneously occupies two binding pockets. Additionally, we solved the 2.06 Å X-ray crystal structures of human Tryptase beta-2 (hTPSB2), in both its apo form and in complex with compound # 1 , experimentally confirming the binding mode and the key molecular interactions predicted by docking studies for this compound. This integrated approach offers a robust framework for elucidating both the strength and mode of interaction of potential tryptase inhibitors.
Organizational Affiliation:
Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Via Mario Negri, 2, 20156 Milano, Italy.